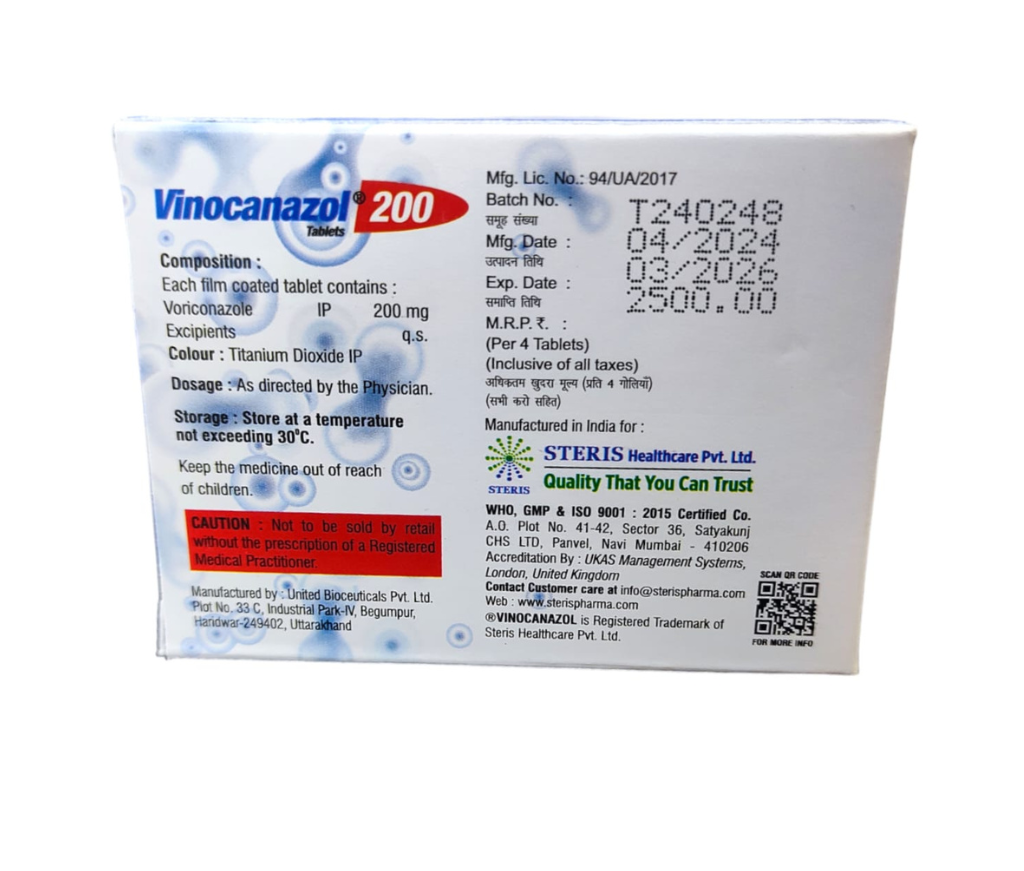
VINOCANAZOL 200
Item requires a valid prescription
Manufactured By Steris Healthcare Pvt Ltd
Composition Voriconazole (200mg)
Rs 1642.03
MRP Rs 2345.75
(30% OFF)
Includes all taxes
Package SIZE
( For 4 Tablet )
100% Authentic
Products
Free
Shipping*
Products
Return Policy
Description:
Voriconazole is a powerful triazole antifungal widely used to treat serious fungal infections. Marketed here as VINOCANAZOL 200 (Voriconazole 200 mg), this medicine is intended for infections that require potent, broad-spectrum antifungal activity. Below you’ll find an SEO-friendly, user-friendly breakdown of what VINOCANAZOL 200 is, how it works, when it’s used, recommended dosing, benefits, safety considerations, and notes about Steris Pharma’s quality and certifications.
What is Voriconazole ?
Voriconazole is a second-generation triazole antifungal. It has broad activity against yeasts (including many Candida species) and moulds (notably Aspergillus species) and is frequently chosen for invasive, potentially life-threatening infections when first-line therapy is needed. The trade name in this article, VINOCANAZOL 200, refers to a tablet formulation containing 200 mg of voriconazole per tablet.
(Reference: clinical pharmacology and product information).
Mechanism of Action —
Voriconazole works by inhibiting lanosterol 14α-demethylase (CYP51), an enzyme in the fungal cell that converts lanosterol to ergosterol. Ergosterol is a crucial component of fungal cell membranes; blocking its synthesis disrupts membrane integrity, increases permeability, and ultimately interferes with fungal growth (generally fungistatic, sometimes fungicidal depending on organism and concentration). This mechanism explains its broad spectrum versus many clinically important fungi.
(Mechanism: triazole inhibition of ergosterol synthesis).
Indications — When VINOCANAZOL Is Used
VINOCANAZOL (voriconazole) is used to treat a variety of serious fungal infections, including but not limited to:
-
Invasive aspergillosis (first-line therapy in many settings).
-
Serious invasive candidiasis (including some fluconazole-resistant species).
-
Scedosporium and Fusarium infections (where other options may be limited).
-
Other deep or disseminated mycoses where voriconazole’s spectrum and tissue penetration are appropriate.
Use is typically guided by culture results, clinical severity, and specialist advice (infectious disease / medical microbiology).
Dosage & Administration
Important: Exact dosing must be individualized by the treating physician, taking patient weight, liver function, drug interactions, and clinical condition into account. The following are commonly referenced dosing patterns used in adults:
-
Loading dose (IV or oral equivalent): Typically 6 mg/kg every 12 hours for two doses (given IV) when rapid levels are needed.
-
Maintenance dose (adults): Common maintenance dose is 200 mg twice daily (i.e., VINOCANAZOL 200 mg tablet every 12 hours) after loading, though some regimens use 4 mg/kg IV q12h for weight-based dosing. For some infections or patient groups, doses up to 300–400 mg twice daily may be used under specialist guidance.
-
Lower-weight adults & children: Dose adjustments are required for patients under certain weight thresholds and for children—pediatric dosing is weight-based.
-
Hepatic impairment: Dose reductions are often required in moderate to severe liver disease.
-
Duration: Varies dramatically—short courses for localized infections; months for invasive disease, sometimes continued until radiologic and clinical resolution plus immune recovery.
Always follow the prescribing information and specialist recommendations; therapeutic drug monitoring can help optimize safety and efficacy for long courses.
Clinical Benefits & Advantages
-
Broad spectrum: Effective against Aspergillus spp., many Candida spp., and several less common moulds — useful when pathogens are resistant or in severe disease.
-
Good tissue penetration: Voriconazole penetrates well into many tissues, including the central nervous system in some cases — important for invasive or disseminated infections.
-
Oral and IV formulations: Allows IV-to-oral step-down therapy, supporting hospital discharge and outpatient continuation with VINOCANAZOL 200 tablets.
-
Proven outcomes: Invasive aspergillosis and certain severe candidiases have better outcomes when treated early with appropriate antifungals like voriconazole under specialist care.
Safety Profile & Side Effects
Voriconazole is effective but associated with several notable adverse effects; monitoring is essential.
Common adverse reactions:
-
Visual disturbances (blurring, altered color perception, photophobia) — often transient but can be distressing.
-
Gastrointestinal: nausea, vomiting, diarrhea.
-
Skin reactions: rash, photosensitivity (sunburn-like reactions), and, rarely, severe cutaneous reactions.
-
Elevated liver enzymes / hepatotoxicity — liver function tests should be monitored.
-
Headache, dizziness, fever.
Serious but less common:
-
Hepatotoxicity — can be severe; discontinue if significant liver injury occurs.
-
QT prolongation / cardiac arrhythmia risks — monitor with interacting medications.
-
Hallucinations / neuropsychiatric effects — reported in some patients.
-
Skin malignancy with long-term photosensitivity — advise sun protection for prolonged courses.
Because voriconazole has many drug–drug interactions (it inhibits several cytochrome P450 enzymes), check concomitant medications carefully — interactions can raise or lower voriconazole levels or affect other drugs.
(Adverse reactions and monitoring guidance).
Drug Interactions & Monitoring
-
Voriconazole interacts with many drugs metabolized by CYP2C19, CYP2C9, and CYP3A4. Examples include certain statins, some antiepileptics, immunosuppressants (tacrolimus, cyclosporine), and warfarin.
-
Therapeutic drug monitoring (TDM) is frequently used for voriconazole to target therapeutic plasma levels and reduce toxicity — especially in prolonged therapy or when interacting drugs are present.
-
Monitor liver function tests, visual symptoms, and ECG (if risk of QT prolongation).
(See specialist prescribing information for comprehensive interaction lists).
About Steris Pharma & Quality Assurance
Steris (Steris Healthcare / Steris Pharma) positions itself as a regulated pharmaceutical marketing/manufacturing entity with standard industry certifications. According to company information, Steris Healthcare / Steris Pharma lists certifications such as WHO-GMP, ISO 9001:2018, and other accreditations claimed on their corporate pages and trade profiles. These certifications generally indicate adherence to recognized quality management and manufacturing practices when valid and up-to-date. Always check the product packaging, certificate downloads, and regulatory documentation to verify current certification status for a specific manufacturing site or batch.
If you’re sourcing VINOCANAZOL 200, ensure:
-
Packaging bears batch number, manufacturing and expiry dates.
-
There is a visible manufacturing license and GMP / WHO-GMP markings where applicable.
-
You purchase from authorised distributors or pharmacies.
-
Official Steris documentation or customer service can provide certificate copies if needed.
(Company certification claims and product quality reminders).
Practical Patient Advice
-
Take as prescribed. Do not stop medication early — fungal infections often need long courses.
-
Follow timing rules. VINOCANAZOL 200 is commonly taken every 12 hours; stick to the schedule for steady levels.
-
Watch for vision changes. If you experience severe or persistent visual symptoms, contact your doctor immediately and avoid driving until evaluated.
-
Avoid excessive sun exposure. Use sunscreen and protective clothing during therapy.
-
Inform your prescriber of all medications. Includes OTCs, herbal products (e.g., St. John’s Wort), and supplements.
-
Laboratory monitoring. Expect periodic blood tests (liver function) and possibly drug level checks for prolonged therapy.
Contraindications & Special Precautions
-
Known hypersensitivity to voriconazole or other azoles.
-
Use caution in pregnancy — assess risks/benefits; alternatives may be preferred.
-
Adjust doses for hepatic impairment and in some cases renal impairment (particularly for IV vehicle sulfobutyl ether β-cyclodextrin accumulation).
-
Genetic variability in CYP2C19 can affect metabolism — some patients (poor metabolizers) have higher drug levels and increased toxicity risk; TDM helps manage this.
(Refer to the full prescribing information for pregnancy categories and detailed contraindications).
Conclusion
VINOCANAZOL 200 is a clinically important, broad-spectrum triazole antifungal used for serious and invasive fungal infections. Its strengths are broad activity and good tissue penetration, and its limitations are a complex interaction profile and notable adverse effects (visual symptoms, hepatotoxicity, skin reactions). When prescribed and monitored by qualified clinicians, VINOCANAZOL 200 can be lifesaving in invasive fungal disease.
If you’re a patient: never self-prescribe — discuss therapy, monitoring, and expected duration with your treating physician. If you’re a healthcare professional: follow local guidelines, consult infectious disease specialists for complex cases, and use therapeutic drug monitoring as appropriate.