Steris Healthcare
Jun 19, 2023
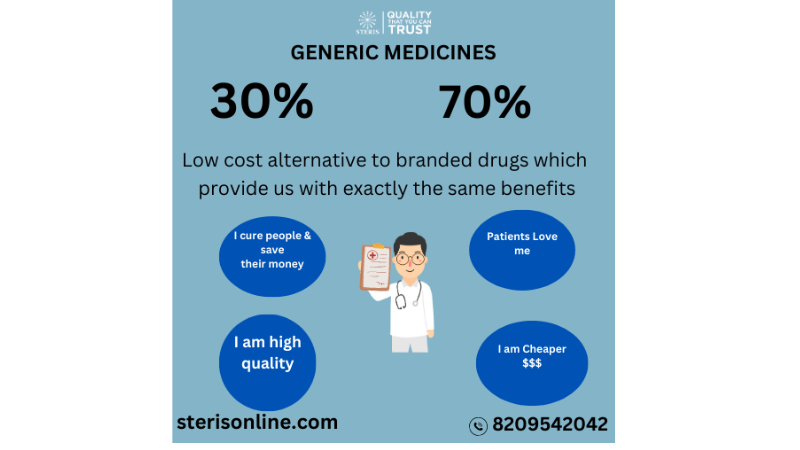
Steris Healthcare is a Generic Brand. Steris provides you Good Quality of Generic Medicines.
Generic medicines, also known as generic drugs or generics, are pharmaceutical products that contain the same active ingredients, dosage form, strength, route of administration, and intended use as brand-name medications. They are typically sold under their chemical or generic names, rather than a specific brand name.
Here are some key points about generic medicines:
Active ingredients: Generic medicines have the same active ingredients as the corresponding brand-name medications. The active ingredient is the chemical component responsible for the therapeutic effect of the drug.
Bioequivalence: Generic medicines must demonstrate bioequivalence to the brand-name drug. This means they must show comparable pharmacokinetic properties (rate and extent of absorption) when administered to individuals under similar conditions.
Safety and efficacy: Generic medicines undergo rigorous testing to ensure they are safe and effective. Regulatory authorities, such as the FDA in the United States, review and approve generic medications based on scientific evidence that demonstrates their quality, safety, and efficacy.
Cost savings: Generic medicines are generally more affordable than brand-name drugs. Generic manufacturers can offer them at lower prices because they don't have to incur the same research and development costs as the brand-name companies.
Appearance and packaging: Generic medicines may look different from their brand-name counterparts in terms of shape, color, and packaging. However, they must meet the same quality and safety standards as the brand-name product.
Prescription substitution: In many countries, pharmacists may substitute a generic medication for a brand-name prescription if it is available. This substitution is typically done with the consent of the prescriber and helps promote cost savings for patients.
Quality control: Generic medicines undergo stringent quality control measures. Generic manufacturers are required to adhere to good manufacturing practices (GMP) to ensure consistent quality, purity, and potency of their products.
For further information please contact:
contact@sterispharma.com
Visit : sterisonline.com
Call : 8209542042
Recent Post

Xylometazoline Hydrochloride Nasal Drops – Fast Relief from Nasal Congestion
Pregabalin and Methylcobalamin Tablets Uses, Benefits & Side Effects
Methylcobalamin Tablets 1500 mcg Uses, Dosage, Side Effects & Benefits Explained
Empagliflozin 25 mg Uses, Dosage, Side Effects, and Benefits

Atorvastatin 10 mg Uses in Hindi | एटोरवास्टेटिन टैबलेट के उपयोग

Atorvastatin 20 mg Uses Explained – Safe Heart, Better Life

Telmisartan 40 mg Uses in Hindi | टैब्लेट के फायदे, साइड इफेक्ट्स और खुराक

Dapagliflozin 10 mg Uses in Hindi: GLIDAPAFLOZIN TRIO 1 FORTE के उपयोग और फायदे
Dapagliflozin 10 mg Uses in Hindi: डापाग्लिफ्लोज़िन 10 मिलीग्राम के उपयोग, लाभ और साइड इफेक्ट्स
Calcium and Vitamin D3 Tablets IP – Bone Health with Vitamin K2, Magnesium & Boron